A trial to confirm if Covid vaccine types can be mixed is underway - what we know so far
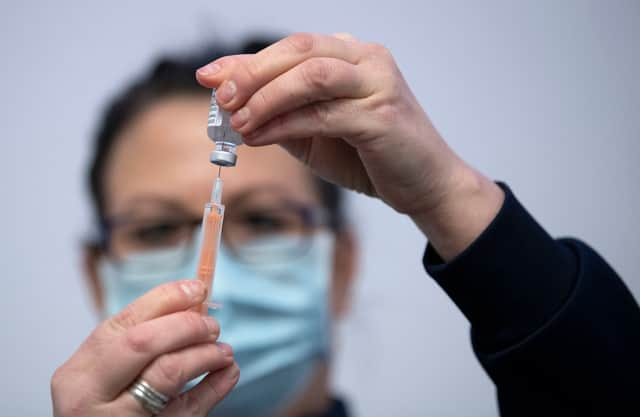

Volunteers are being recruited to take part in a world first study to examine the effects of mixing and matching Covid-19 vaccines.
The trial will be conducted on more than 800 participants over the age of 50, and will compare the effects of issuing one dose of the Oxford/AstraZeneca vaccine and one dose of the Pfizer/BioNTech vaccine.
Advertisement
Hide AdAdvertisement
Hide AdThe intervals between the doses will be either four or 12 weeks, in order to test the effects of waiting longer between jabs on the immune response.
‘No current plans to change vaccination programme’
In a statement from the Government announcing the study, it explains that a “same-dose regimen is currently implemented for the national Covid-19 vaccination programme, and there are no current plans for this to change”.
This means that anyone who received either the Oxford/AstraZeneca vaccine or the Pfizer/BioNTech vaccine will not be affected by the study, and will receive their second dose from the same vaccine over the same 12 week interval.
The study is being run by the National Immunisation Schedule Evaluation Consortium (NISEC) across eight National Institute for Health Research (NIHR) supported sites.
Advertisement
Hide AdAdvertisement
Hide AdThese eight sites are situated in locations across England, in London (St George’s and UCL), Birmingham, Liverpool, Oxford, Southampton, Bristol and Nottingham.
Patients for the study will be recruited over the course of February, with volunteers recruited via the NHS Covid-19 Vaccine Research Registry. Vaccinations are expected to start towards the middle of the month, with initial results made available over the summer period.
Members of the public can volunteer to take part in the study, and further vaccine studies, by joining the registry.
Official guidance advises against mixing vaccines
Official advice from the Joint Committee on Vaccination and Immunisation (JCVI) states that “the second vaccine dose should be with the same vaccine as for the first dose”.
Advertisement
Hide AdAdvertisement
Hide Ad“Switching between vaccines or missing the second dose is not advised as this may affect the duration of protection,” the JCVI adds.
Only in very rare circumstances should a different vaccine be used.
The Greenbook states: “Every effort should be made to determine which vaccine the individual received and to complete with the same vaccine.
“For individuals who started the schedule and who attend for vaccination at a site where the same vaccine is not available, or if the first product received is unknown, it is reasonable to offer one dose of the locally available product to complete the schedule.
Advertisement
Hide AdAdvertisement
Hide Ad“This option is preferred if the individual is likely to be at immediate high risk or is considered unlikely to attend again.”
‘Improving the flexibility of vaccine delivery’
Lead researcher Dr Matthew Snape, associate professor in paediatrics and vaccinology at the University of Oxford, said that the study is driven by improving the "flexibility in vaccine delivery”.
Speaking to the BBC Radio 4 Today programme, he said: “If somebody turns up to have their second vaccine, and they’ve already received say the Pfizer vaccine and it’s not available that day, then can they receive the Oxford vaccine as an alternative? And vice versa, of course.
“That would greatly improve the flexibility of delivery.
“It is good medicine to make sure you have flexibility in what you can do and that you’re protecting against any future problems.
Advertisement
Hide AdAdvertisement
Hide Ad“We’re looking to see if immunising with a mixed schedule is as good as immunising with the straight approved schedule.
“There’s also some potential advantages: in animal studies for example, we see a better antibody response with a mixed schedule rather than the straight schedule.
“This is new and this is exciting, it will be the first study looking at using the RNA vaccine, which is the Pfizer/BioNTech one, and a viral vector vaccine, which is the Oxford/AstraZeneca one, in the same schedule.”
‘Another exciting step forward’
National Clinical Lead for the NIHR Covid Vaccine Research Programme, Professor Andrew Ustianowski, said: “This is another exciting step forward in finding a variety of vaccine options for the UK and globally.”
Advertisement
Hide AdAdvertisement
Hide AdInterim Chair of the Government's Vaccines Taskford, Clive Dix, added: “This study will give us valuable insight into how vaccines work together and could give us more flexibility as we continue to tackle this virus in the weeks, months and years ahead.
“This is yet another example of the UK leading the way in vital research into Covid-19 - and something that people both in this country, and around the world, could benefit from.”